Source: https://www.undercurrentnews.com
STAVANGER, Norway — Antimicrobial resistance (AMR) is a global megatrend, and the attention on the use of antibiotics in raising animals will only increase in the coming years, warned Nutreco CEO Knut Nesse.
The World Health Organization confirmed last year that one cause of AMR — the process by which a microbe evolves to become more or fully resistant to antimicrobials which previously treated it — was the inappropriate use of antibiotics in animal husbandry.
On top of the inherent risk posed by this irresponsible antibiotic use, as AMR continues to become a megatrend, it will attract increasing attention from media and consumer pressure, he believes.
“The aquaculture industry needs to respond to this challenge in a proactive way through innovation,” Nesse told the audience of aquaculture executives at the recent Aquavision 2016 conference.
He called for cross-collaboration across the entire aquaculture value chain, and said that part of the answer will come from preventative health, and innovative nutritional, solutions.
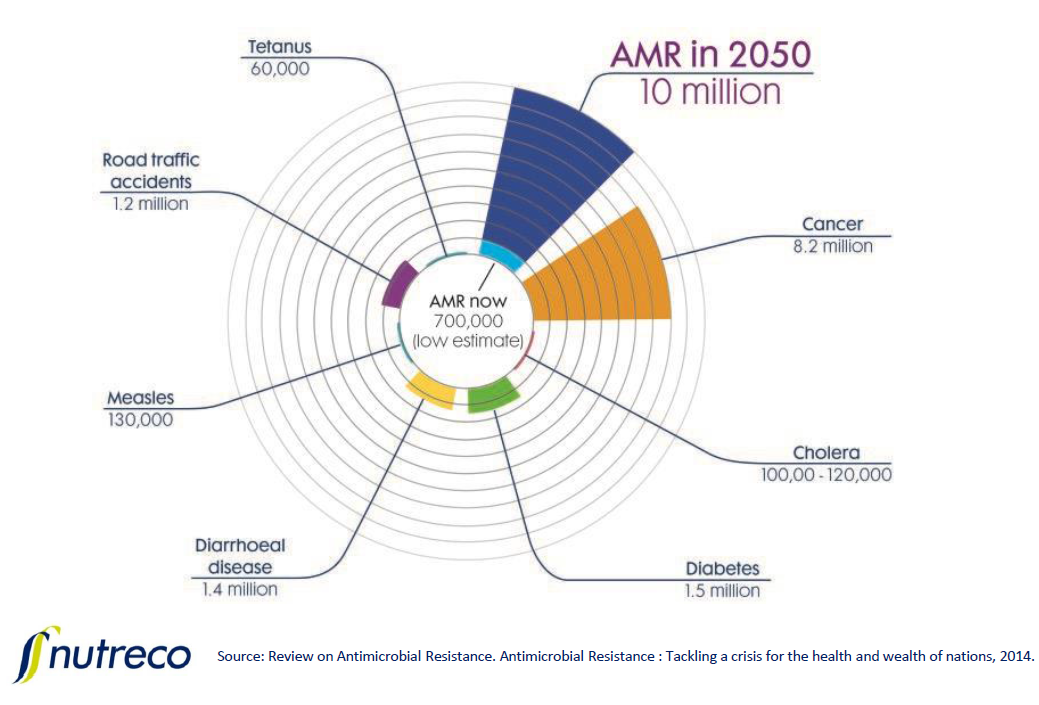
Nesse presented a diagram which predicts AMR could be the leading cause of deaths globally by 2050, contributing 10 million deaths per year.
He also noted headlines from a range of food sectors showing the global trend for retailers to reject animals raised with antibiotics.
Costco Wholesale is to phase out drugs important in human medicine from its supply chain; Tyson and Chick-fil-A (the largest US buyer of chicken) are to distance themselves from drug use in chickens; and Walmart has pressed its meat suppliers on antibiotic use.
“The public perception in some areas already is that aquaculture is becoming more unsustainable,” he said. “Antibiotic use is part of that picture. As an industry we must react pro-actively.”
Nesse predicts that in the future the industry will operate under global targets to reduce antibiotic use in food production to an agreed level per kilogram of livestock and fish. He also expects there to be special restrictions on the use of those drugs important in human medicine.
There will, he added, also be full transparency over antibiotic use in animal husbandry. The sector has probably seen the first step in this process just this month, as the Chilean government, under legal pressure from environmental groups, published the amount of antibiotic use per company for the first time ever.
Costco’s switch the eye-opener
In 2015 US club store Costco made the decision to switch its majority salmon supply from Chile to Norway.
Chile had provided Costco with 90% of its farmed Atlantic salmon, and Norway just 10%; but starting in June 2015, Norwegian, antibiotic-free salmon was to fulfill about 60% of its needs.
The store purchased 600,000 pounds of salmon filets a week — close to 10% of all US Atlantic salmon imports from Chile.
Walmart weighed in, updating its policy to state that antibiotics should “only be used for medical purposes” and that suppliers should “eliminate the growth promotion uses of antibiotics”.
SalmonChile’s chairman, Felipe Sandoval, stated in response that exported Chilean salmon products did not have traces of antibiotics. “The final products from Chile don’t have traces of antibiotics, otherwise they could not enter other markets.”
According to Sandoval, there were no “concrete facts” of the existence of residues of antibiotics in Chilean salmon. “Maybe other competitors want to take advantage of things that are not so certain,” he said.
Some producers, including Australis Seafoods, Cermaq and Nova Austral, have set salmon farms in the Magallanes region at the tip of Chile, where they have no need to use antibiotic treatment. The region is more than 1,000 miles south of Puerto Montt, the traditional hub of Chile’s salmon farming industry.
But the growing trend to market salmon free of antibiotic treatment could damage Chile’s reputation, which in April 2016 was still struggling to shake off a negative image stemming from the outbreak of infectious salmon anemia a decade ago, according to Cermaq CEO Jon Hindar.
Chile’s salmon industry as a whole had in 2015 tabled the idea of promoting its “from Patagonia to the table” brand, in response to concerns over extensive use of antibiotics.
Pesquera Camanchaca, meanwhile, reduced its use of antibiotics in 2015, after implementing its health management program. Despite a 10% higher salmon harvest at 43,000 metric tons, the company decreased its use of antibiotics by 30%.
Speaking at Aquavision, Norwegian fish health firm Pharmaq revealed it has its live salmon rickettsial septicaemia vaccine testing across 20 million young Chilean fish, after the innovative product was approved in February.
“The product was commercially launched in Chile with an approval in February 2016,” Martinsen told Undercurrent. “The product is therefore in commercial use, but we are lacking the field efficacy performance as the product has been approved based on laboratory data (as all new vaccines in Chile are).”
“We expect to collect more data throughout the rest of this year and continue to get more data from commercial operations also next year.”